Background
Maplirpacept (PF-07901801) is an anti-CD47 molecule designed to enhance phagocytosis and antitumor activity by preventing CD47-mediated inhibitory signaling. Maplirpacept currently is being evaluated in an ongoing, multi-center, two-part, Phase 1a/1b study in participants with advanced hematologic malignancies (NCT03530683). Early safety and efficacy results for maplirpacept monotherapy have previously been reported in patients with refractory or relapsed lymphomas (Patel et al. Blood 2021;138:3560).
Study Design
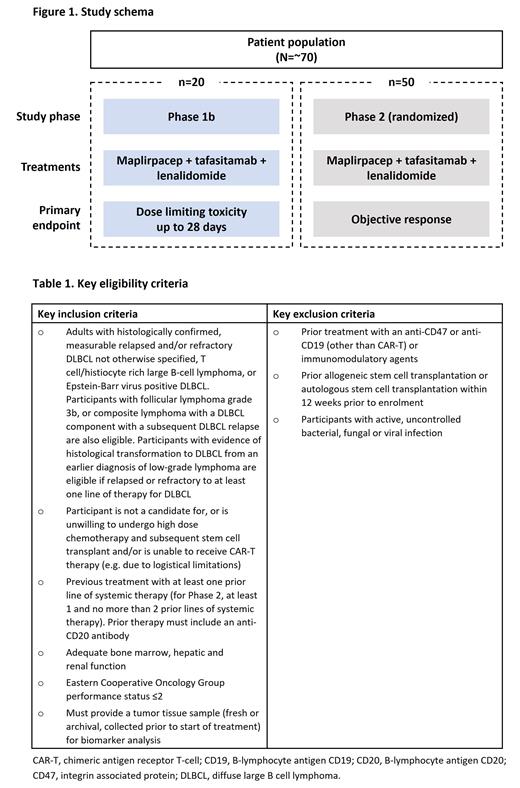
This trial is a multicenter, open-label, Phase 1b/2 study (NCT05626322), to evaluate the effects of maplirpacept, in combination with tafasitamab and lenalidomide in participants with relapsed or refractory diffuse large B-cell lymphoma (DLBCL). The estimated enrollment is 70 patients, and the study will be split into two parts (Figure 1). Phase 1b will determine the maximum tolerated dose or recommended Phase 2 dose (2 dose levels) of maplirpacept, in combination with approved doses of tafasitamab and lenalidomide. In Phase 2, participants will be randomized to high or low doses of maplirpacept, in combination with approved doses of tafasitamab and lenalidomide, as per the Oncology Center of Excellence ‘Project Optimus’ initiative for dose optimization and selection in oncology drug development.
Study interventions will be administered in 28-day cycles. Maplirpacept will be given weekly, by intravenous (IV) infusion, for the first three cycles and subsequently every two weeks. Tafasitamab will be administered, by IV infusion, on Days 1, 4, 8, 15 and 22 in cycle 1, weekly in cycles 2 and 3, then every 2 weeks in cycle 4 and beyond. Lenalidomide will be taken orally, daily for Days 1 to 21 of each 28-day cycle, for the first 12 cycles.
In Phase 1b, approximately 20 participants will be allocated to sequential doses of maplirpacept, co-administered with approved doses of tafasitamab and lenalidomide, to select doses for further assessment in Phase 2. The primary endpoint for Phase 1b is to assess dose limiting toxicities up to 28 days following the first dose. In Phase 2, approximately 50 participants will be randomized to 1 of 2 different doses of maplirpacept, co-administered with tafasitamab and lenalidomide. The primary endpoint for Phase 2 is objective response as per the Lugano Response Classification Criteria 2014.
Secondary endpoints are frequency of adverse events, frequency of clinical laboratory abnormalities, objective response (Phase 1b only), complete response, duration of response, duration of complete response, progression free survival, maplirpacept, tafasitamab and lenalidomide PKs, and immunogenicity of maplirpacept and tafasitamab.
Inclusion criteria (Table 1) are adults with histologically confirmed, measurable relapsed and/or refractory DLBCL not otherwise specified, T cell/histiocyte rich large B-cell lymphoma, or Epstein-Barr virus positive DLBCL according to the 5th edition of the WHO classification of lymphoid neoplasms. Additionally, participants with follicular lymphoma grade 3b, or composite lymphoma with a DLBCL component with a subsequent DLBCL relapse are also eligible. Participants with evidence of histological transformation to DLBCL from an earlier diagnosis of low-grade lymphoma are eligible if relapsed or refractory to at least one line of therapy for DLBCL. Further inclusion criteria are participants who are not a candidates for, or are unwilling to receive, high-dose chemotherapy and subsequent stem cell transplant and/or are unable to undergo chimeric antigen receptor T-cell (CAR-T) therapy (e.g. due to logistical limitations), treatment with at ≥1 prior line of systemic therapy (for Phase 2, at least 1, and no more than 2, prior lines of systemic therapy) and prior therapy must include an anti-CD20 antibody. Participants must have adequate bone marrow, renal and hepatic function and an Eastern Cooperative Oncology Group performance status ≤2. Participants must provide a baseline tumor tissue sample (fresh or archival), for biomarker analysis.
Exclusion criteria include previous treatment with anti-CD47, anti-CD19 (other than CAR-T) or immunomodulatory agents, prior allogeneic stem cell transplantation or autologous stem cell transplantation within 12 weeks prior to enrolment, and participants with active, uncontrolled viral, bacterial or fungal infections.
Disclosures
Hoyle:Pfizer Inc: Current Employment, Current holder of stock options in a privately-held company. Paccagnella:Pfizer Inc: Current Employment, Current holder of stock options in a privately-held company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal